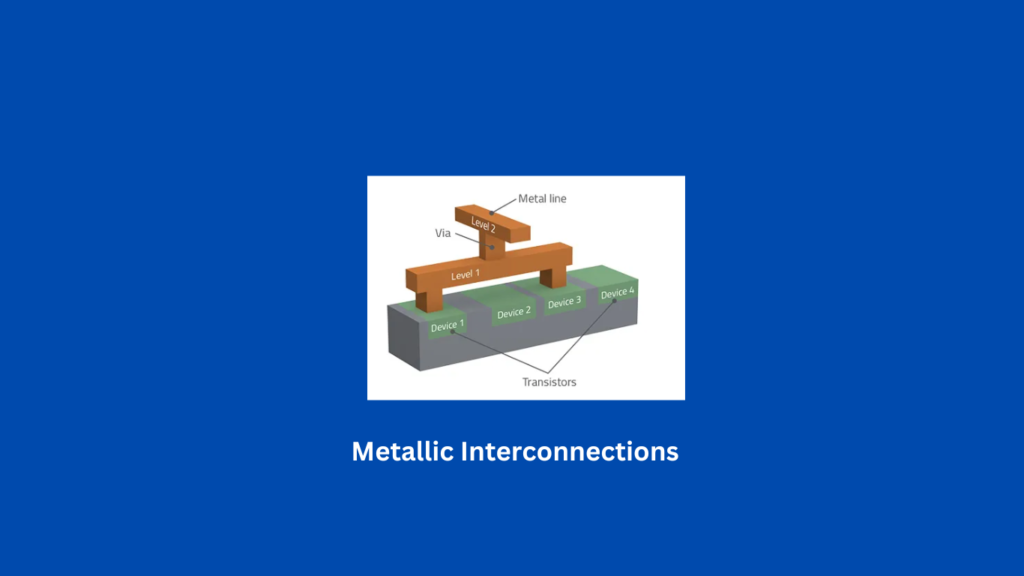
Metallic interconnections are widely used in electrical systems because they effectively carry electric current and support information transfer. Here’s why:
Metallic interconnections
I find it fascinating that metallic conductors, like copper and aluminum, are so effective. These metals consist of positively charged ions arranged in a regular lattice, with free electrons moving around this lattice. This arrangement allows the free electrons to travel at high speeds, which is crucial for conducting electricity. However, the electrons move randomly without an overall direction until an electric force field is applied.
Behavior of Electrons
When an electric field is applied to a metallic conductor, the free electrons start to move in one direction. This movement creates an electric current. At very low temperatures, the lattice doesn’t vibrate, so electrons move almost unimpeded. But as the temperature increases, the lattice vibrates more, causing the electrons to collide and creating electrical resistance. This resistance limits the current, which is why Ohm’s Law (V = IR) is fundamental in understanding how electrical circuits work.
We also need to consider that the speed of electromagnetic (EM) waves in conductors is much slower than in air. This is because the electrons reradiate EM waves in all directions, which affects the propagation speed of these waves.
You might also be interested to know that the movement of charge in conductors creates magnetic fields and stores magnetic energy, described by inductance, while localized charge creates electric fields and stores electric energy, described by capacitance. The balance between these energy storage forms and how quickly energy can be transferred affects the performance of transmission lines.
Ultimately, understanding how energy is transferred through conductors and how interruptions can degrade signal integrity is crucial for designing efficient integrated circuits (ICs) that use minimal power. By grasping these concepts, you can better appreciate how electrical systems manage information and energy.
How Interconnect Materials Evolved
If you’ve ever wondered how signals move inside a chip, the answer lies in interconnect materials. For a long time, aluminum was the go-to choice. We used to deposit a layer of aluminum, pattern and etch it, and then insulate the metal lines to separate them. This approach worked well for decades.
But in the late 1990s, things changed. We shifted to copper interconnects because copper conducts electricity better—kind of like how a copper-bottomed pan heats up faster than an aluminum one. With copper, we saw a clear boost in overall chip performance. Not only did it improve conductivity, but it also allowed us to shrink the metal lines, helping us keep up with transistor scaling.
You’ll also find that copper is more reliable and durable than aluminum. However, working with copper wasn’t as simple. We had to rethink the entire manufacturing process. It starts with depositing a dielectric layer like silicon dioxide, creating trenches, and then filling them with copper using electroplating or chemical deposition. After that, any excess copper is removed to create a smooth surface for the next layer.